3D Cell Models Derived from Patient Tumors – A New Approach to Accelerate Identification of Drug Targets and Testing
Tumor models are powerful tools in the development of cancer drugs
Many cancers can be cured if detected early and treated effectively but the attrition rate of cancer drugs is very high [1]. This is often due to safety issues, or lack of efficacy in clinical trials. Picking out promising candidates early is therefore crucial, and tumor models play an essential role in this selection process.
In vitro and in vivo models have been invaluable in cancer therapy development, drug screening, and basic research into the molecular mechanisms of tumor growth and metastasis. But ensuring time and cost-effective drug development means that modeling must go up a level to resemble the complexity of human tumors more closely [2]. This involves transitioning from two-dimensional (2D) to three-dimensional (3D) [3].
Adding another dimension to cell models sharpens a predictive tool
2D monolayer cell cultures fail to imitate many aspects of tumor architecture and microenvironments. These limitations have driven the development of 3D models that more accurately recapitulate the cellular composition of tumors. 3D models have many advantages over 2D models [3], including:
- The natural cell morphology is preserved, and cells grow into multiple layers
- The 3D form mimics the access to nutrients in tumors, with core cells often inactive
- Cells are well differentiated
- Cell junctions are common
- 3D models are more resistant to drugs and with better metabolism to give a more accurate representation of drug effects
- Cell proliferation rates are realistic and can be modulated
- Gene and protein expression levels resemble those of cells in vivo
- Levels of resistance to drug-induced apoptosis are higher
- Cells respond more realistically to mechanical stimuli
Overall, 3D cell cultures increase the dimensionality of cell-cell interactions that are fundamental to generating a phenotype predictive of in vivo biology but performed in vitro [4]. As a result, 3D models are a better predictive tool for drug discovery compared to 2D models, and show great promise in guiding personalized medicine, including immunotherapy [5,6,7].
3D cell models can take many forms
Spheroids are relatively simple 3D cell models derived from cell lines, multicellular mixtures, primary cells, tumor cells and tissues.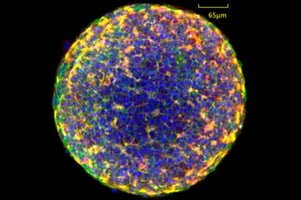
Organoids are more complex than spheroids and have been defined in many ways [8]. One definition is a self-organized cell aggregate derived from primary tissue or mesenchymal stem cells (MSCs) capable of self-renewal, typically organized in 3D constructs that replicate the complex structure of an organ and mimic its in vivo physiology [7].
Tumoroids are essentially “tumor-like organoids” that are typically prepared using cells from primary tumors harvested from patients.
Tumoroids – highly translational tools that give new insights into cancer
Tumoroids can recapitulate the complex genetic and molecular compositions of solid cancers, making them extremely valuable in the study of disease progression, the identification of drug targets, and drug testing in general. At the level of the individual, a tumoroid based on a patient’s tumor cells can be used in personalized medicine to predict the response of specific tumors to therapy.
One series of proof-of-concept studies demonstrates the potential for high-resolution image-based analysis of 3D spheroid models for drug discovery applications that could soon be routine practice in drug discovery workflows [9]. The studies included studying real-time immune cell interactions in a multicellular 3D lung cancer model and using a 3D model of gastric carcinoma in a high throughput screening application to determine dose-dependent drug efficacy.
Another example examines factors affecting human glioblastoma angiogenesis and shows the power of tumoroids in mimicking complex tumor microenvironments [10]. The model is scalable, easy to control, cost-effective, and can be used as a preclinical model to study microenvironment cues of tumor angiogenesis.
The value of tumoroids in personalized medicine is illustrated by a study on lung cancer [11]. A cancer form that shows substantial genetic and phenotypic heterogeneity across individuals, making it particularly interesting as a target for personalized medicine. Tumoroids and normal bronchial organoids were quickly established from patient tissues and recapitulated the original tissue architecture and genomic alterations even after extensive in vitro expansion. The tumoroids also responded to drugs based on their genomic alterations, making them a powerful tool for predicting patient-specific drug responses.
How to smoothen the transition to 3D
There are clearly many good reasons for transitioning from 2D to 3D, but 3D cell cultures are expensive and can be a challenge to maintain and analyze. At the same time, the high-quality data they generate can motivate the transition, as can the possibility of avoiding the use of animal models.
Ready to enter the next level?
Please fill out this form and contact our experts today to find the perfect solution for you!
Don't miss our regular updates on scientific topics around 3D Cell Culture
References
[1] Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164.[2] Tatullo M, Marrelli B, Benincasa C, Aiello E, Makeeva I, Zavan B, Ballini A, De Vito D, Spagnuolo G. Organoids in Translational Oncology. J Clin Med. 2020 Aug 27;9(9):2774. doi: 10.3390/jcm9092774. PMID: 32867142; PMCID: PMC7564148.
[3] Jensen C, Teng Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front Mol Biosci. 2020 Mar 6;7:33. doi: 10.3389/fmolb.2020.00033. PMID: 32211418; PMCID: PMC7067892.
[4] Souza GR, Spicer T. SLAS special issue editorial 2022: 3D cell culture approaches of microphysiologically relevant models. SLAS Discov. 2022 Apr;27(3):149-150. doi: 10.1016/j.slasd.2022.03.006. Epub 2022 Mar 24. PMID: 35339725
[5] Xu H, Lyu X, Yi M, Zhao W, Song Y, Wu K. Organoid technology and applications in cancer research. J Hematol Oncol. 2018 Sep 15;11(1):116. doi: 10.1186/s13045-018-0662-9. PMID: 30219074; PMCID: PMC6139148.
[6] Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. https://doi.org/10.3390/cancers13040874
[7] Tatullo M, Marrelli B, Benincasa C, Aiello E, Makeeva I, Zavan B, Ballini A, De Vito D, Spagnuolo G. Organoids in Translational Oncology. J Clin Med. 2020 Aug 27;9(9):2774. doi: 10.3390/jcm9092774. PMID: 32867142; PMCID: PMC7564148.
[8] Simian M, Bissell MJ. Organoids: A historical perspective of thinking in three dimensions. J Cell Biol. 2017 Jan 2;216(1):31-40. doi: 10.1083/jcb.201610056. Epub 2016 Dec 28. PMID: 28031422; PMCID: PMC5223613.
[9] Wardwell-Swanson J, Suzuki M, Dowell KG, Bieri M, Thoma EC, Agarkova I, Chiovaro F, Strebel S, Buschmann N, Greve F, Frey O. A Framework for Optimizing High-Content Imaging of 3D Models for Drug Discovery. SLAS Discov. 2020 Aug;25(7):709-722. doi: 10.1177/2472555220929291. Epub 2020 Jun 2. PMID: 32484408.
[10] Tatla AS, Justin AW, Watts C, Markaki AE. A vascularized tumoroid model for human glioblastoma angiogenesis. Sci Rep. 2021 Oct 1;11(1):19550. doi: 10.1038/s41598-021-98911-y. PMID: 34599235; PMCID: PMC8486855.
[11] Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, Choi EK, Jeong SY, Taylor AM, Jain S, Meyerson M, Jang SJ. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019 Sep 5;10(1):3991. doi: 10.1038/s41467-019-11867-6. PMID: 31488816; PMCID: PMC6728380.
