Transwell assays for cell migration and invasion: sometimes you can’t beat a good classic
In vitro cell migration and invasion assays are indispensable tools in the quest for better biomarkers, therapeutic targets and agents to intervene in complex phenomena like cancer metastasis, pathological angiogenesis and inflammatory diseases. While there is a wealth of options for quantifying cell migration and invasion, the “tried and true” transwell assay (also known as the Boyden chamber assay, filter assay, or transmembrane assay) remains one of the most popular choices. In this blog article, we explore why the humble Boyden chamber and its many variations are still going strong—especially for applications in drug discovery.
The transwell assay principle - there is beauty in simplicity
The transwell concept dates back to the early ‘60s, when Stephen Boyden devised a specialized culture chamber to study leukocyte chemotaxis [1]. The principle is simple: a porous membrane insert or filter separates the culture dish into two medium-filled compartments. As cells seeded into the upper compartment migrate toward a chemo-attractant source in the lower compartment, they must actively migrate through pores of a defined size—one that is too small to permit passive cell transit. The better the cells are at migrating along the chemoattractant gradient established in the assay chamber, the more of them will make their way through to the other side of the membrane during the incubation period. Simple, but clever—because as we shall see this elegant design is highly enabling.
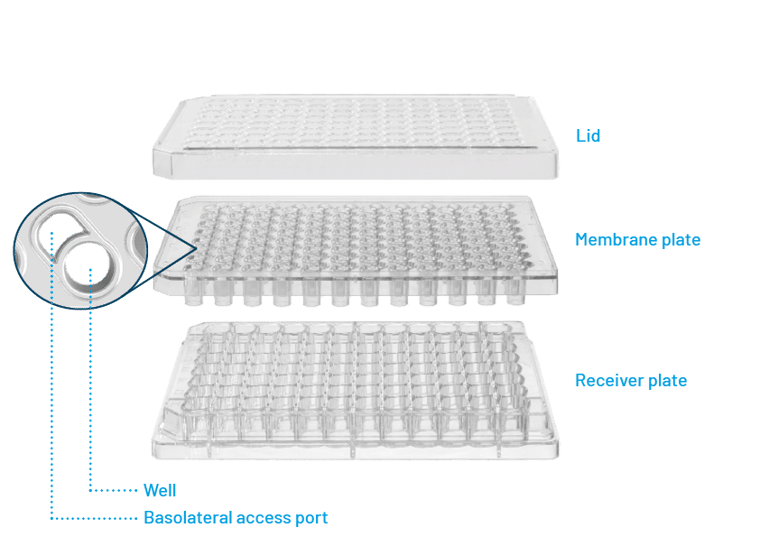
Figure 1: Design of membrane insert plate system
Transwell assays are relatively inexpensive end-point assays that can readily be scaled and automated for higher throughput applications. They are reasonably easy to set up, perform and analyze with standard lab equipment, and can deliver quantitative, reproducible results in just a few hours. Analysis is straightforward: typically, the migrated cells are fluorescently labeled with a live-cell dye like Calcein-AM, detached from the underside of the membrane, and quantified in a standard fluorescence microplate reader.
Why choose transwell assays for high-throughput screening?
A frequent bottleneck in drug discovery occurs after large-scale primary screening, when investigating promising targets and drug candidates to confirm their suitability for further development.
One contributor to this bottleneck is the increasing trend toward more physiologically relevant cell-based assays and phenotypic studies, which can be time-consuming, labor-intensive and challenging to configure for high-throughput.
In recent years, much progress has been made in assay miniaturization, scale-up and automation technologies to improve throughput and cost-efficiency of in vitro cell-based assays. Assays for cell migration and invasion are no exception. A number of solutions for quantifying migration-invasion have been adapted for higher throughput.
As well as transwell assays, these include scratch (wound-healing) assays, exclusion zone assays, and microfluidics arrays. While all of the options have both pros and cons for drug discovery applications [2], the unique combination of advantages offered by transwell assays makes them particularly attractive:
1. High physiological relevance
Transwell assays can be tailored to mimic the complex environments cells encounter in vivo, enhancing the relevance of the assay results. In addition to quantifying cell migration, the two-compartment system can easily be adapted to evaluate invasive potential by coating the membrane with a gel that mimics the extracellular matrix (ECM).
The ability of cells to penetrate this barrier reflects whether the underlying machinery for cell-matrix interactions, proteolysis of ECM components and migration through the matrix is present and functional
2. Versatility
The two-compartment design makes the transwell assay one of the most versatile options available. Various agents and test substances can be added to the upper compartment to modulate intracellular signalling pathways, cell surface receptors, or cell-matrix interactions.
At the same time, chemoattractants, inhibitors or other agents can be added to the lower chamber. By running the same assay conditions with and without an ECM layer, effects on cell invasiveness can be normalized for migratory effects with a ratiometric calculation. Another modification involves establishing a confluent cell monolayer on one side of the membrane to mimic a tissue barrier—e.g., for transepithelial migration assays.
With this high degree of flexibility, the same basic format can be used to explore a wide variety of potential targets, therapeutic candidates and factors affecting cell adhesion, migration and invasion. With a range of membrane pore sizes and densities to choose from, there is also scope to optimize for different cell types.
3. Gradient-directed motility
One of the stand-out features of the transwell format is the ability to establish transient gradients by adding chemoattractant or ECM proteins to the lower compartment. This makes it possible to analyze chemotactic and haptotactic responses, which play crucial roles in progression of many diseases, most notably cancer cell invasion and metastasis.
Chemokinesis (random migration in the absence of a gradient) can also be assessed by adding equal concentrations of substance to the upper and lower compartments.
4. Scalability
Membrane inserts for transwell assays now come in a wide range of well densities. Automation-friendly 96-well formats, such as Greiner Bio-One’s new ThinCert® 96 well HTS insert, are particularly enabling for higher throughput studies.
With more wells you can include more compounds, treatment conditions and replicates on the plate. This significantly improves cost-efficiency and screen quality, without the added risks, difficulties and cost of working with the very low volumes required for higher well-density microplates or microfluidics devices.
At the same time, reduced working volumes translate into lower consumption of expensive reagents and precious samples. If you happen to be working with rare cell lines or primary patient-derived cells, this can make a huge difference to the cost and feasibility of your study.
The ability to move seamlessly from a lower well-density to a higher one, without significantly changing the assay format, can also simplify and accelerate assay development and validation.
Putting it into practice
Another advantage of transwell technology is that with its long history there has been plenty of time to develop optimized devices and protocols to address a wide range of applications. In particular, there have been some great advances in the technologies and protocols for scaling transwell migration assays for high-throughput.
In the next article we’ll take a closer look at a typical 96-well cell migration assay protocol and explore some of the key factors for successful implementation.
References
[1] Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. (1962) 115:453-466
[1] Kramer N et al. In vitro cell migration and invasion assays. Mutation Research/Reviews in Mutation Research (2013) 752(1):10-24.
