Transwell migration assays for HTS - how to maximize your success
Among the many assay technologies available for quantifying cell migration and invasion, the classic transwell migration assay (also known as a Boyden chamber or filter assay) is one of the best options for mimicking a variety of in vivo conditions without too much complexity or expense.
However single-insert solutions reach their limits when large-scale studies in high-throughput need to be performed. Now, with the new ThinCert® HTS 96 well insert, high-throughput cell migration assays are more reproducible, less error-prone, and easier to automate than ever before.
In this article, we walk you through a typical assay protocol to see how you can maximize success of transwell migration and invasion assays with the new 96 well HTS insert
A simplified workflow for HTS migration assays
As detailed in the previous article of this series, a Boyden chamber consists of two compartments separated by a semi-permeable membrane through which cells migrate. With the option to establish ECM substrates and cell cultures on the membrane, the chamber can also be adapted for cell invasion assays and assessment of migration across cell barriers (e.g., transendothelial migration assays).
To scale-up this concept for high-throughput, the ThinCert® HTS insert incorporates 96 wells on a single porous membrane plate manufactured entirely from polycarbonate. When nested precisely within a matched polystyrene receiver plate, the insert creates 96 individual migration chambers, each with an upper compartment for cultured cells and a lower compartment for chemoattractant. Generously sized ports adjacent to each chamber allow multichannel pipettes and liquid handling robots easy access to the lower compartment, without the need for disassembly. This elegant, 2-component design makes for a simplified, automation-friendly workflow.
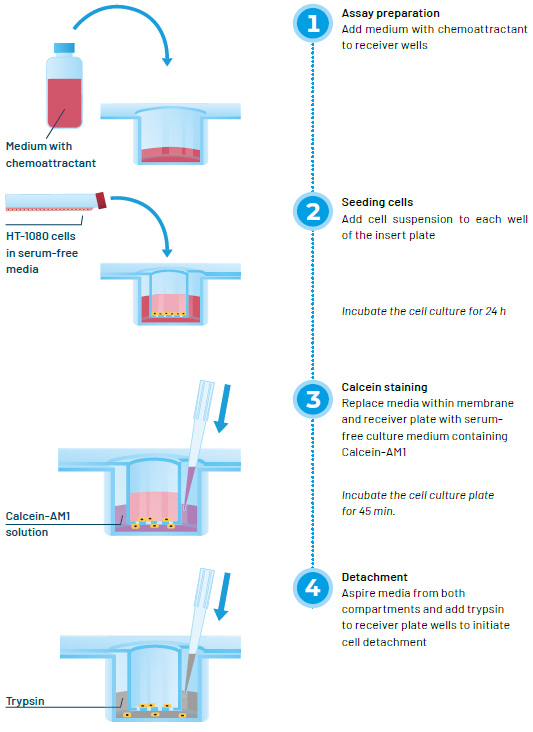
To set up an assay, culture medium with chemoattractant is dispensed into the receiver wells. After adding cell suspension to each well of the membrane plate, the assembled device is incubated for a defined period to allow cells to migrate through the membrane pores. At the end of the incubation period, medium in the lower compartments is replaced with serum-free medium containing Calcein-AM1 to stain live cells. Following a 45-minute incubation, medium is aspirated from both compartments, and trypsin solution is added to the lower compartment to detach migrated cells adhering to the underside of the membrane. Once the cells have been detached, the insert is removed and signal in the receiver wells is quantified with a fluorescence plate reader.
Choosing the right pore size
One of the first and most crucial considerations when selecting a membrane insert is the pore diameter. The size of the pores must be large enough for cells to actively squeeze through, but small enough to prevent their passive transit. The optimal size will depend not only on how large the cells are when adhered to the membrane, but also on the size, shape and deformability of their nuclei.
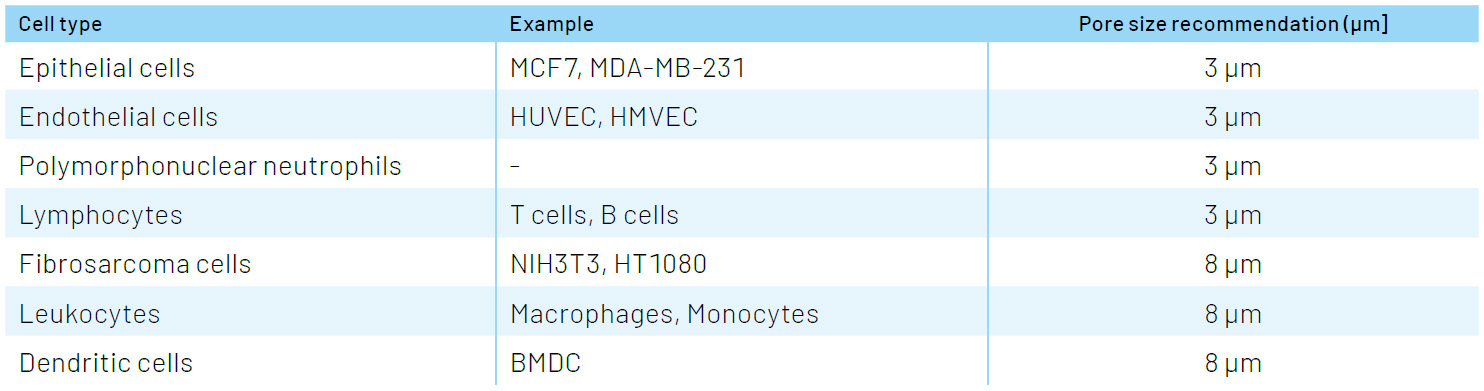
While there is plenty of published literature on the size of different cell types and their nuclei, a general rule of thumb is to start with a pore size slightly larger than the nucleus. Pore sizes for ThinCert® HTS inserts have been strategically chosen to cover a wide variety of migratory cell types: the 3 µm pore is ideal for lymphocytes and other cells with small nuclei, while the 8 µm size is suitable for most epithelial and fibroblast cell types.
Selecting the right pore size for common cell types:
Taking control of assay development
While it’s easy to get up and running quickly with a transwell assay, time spent on assay design and optimization pays big dividends in terms of assay performance and reproducibility.
In addition to all the usual guidelines for best practice in assay development, here are a few key considerations specifically for migration and invasion assays:
1. Seeding density
The density of pores in the membrane insert has a direct bearing on the optimal cell seeding density. The higher the seeding density, the more likely it is that cells will settle directly over membrane pores. Eventually, at very high densities, so many cells settle on pores that it becomes impossible to distinguish chemokinetic effects (random transmigration due to increased motile activity) from chemotactic or haptotactic effects (migration stimulated by the chemoattractant or ECM gradient).
Knowing the pore density and the growth area of the membrane can help you optimize the seeding density such that most of the cells (say 95%) will have to travel to a pore. In this way assay “noise” from unstimulated cells is reduced. At the other extreme, when the seeding density is too low, cells may have to travel long distances to find a pore and too few cells make it through the pores over the time course of the experiment. In this case, assay sensitivity decreases.
2. Controls
To correct for unstimulated random migration, negative control wells with no chemoattractant are essential, and ideally should be present on every plate of a screen or large study. Likewise, positive controls and/or a dose-response series with a known chemoattractant is essential during assay development to calculate quality metrics such as Z’ factor and optimize the assay window.
To control for chemokinesis, equal concentrations of chemoattractant can be added to both the upper and lower compartments. If available, inclusion of a non-migratory cell type for comparison can be informative. In the case of invasion assays, a ratiometric measurement of migration with and without ECM gel may improve assay quality.
3. Migration incubation time, assay linearity and dynamic range
Since different cell types migrate at different rates on different surfaces, the migration incubation time and chemoattractant gradient should be optimized for each cell type and substrate used. For optimal performance and quantitative results with this HTS-friendly assay protocol, it is important to ensure that the fluorescent signal (RFU) correlates with the number of migrated cells at a fixed concentration of chemoattractant.
The optimal conditions will depend on a number of parameters, including cell type, pore size and density, cell seeding density, migration incubation time and the Calcein staining conditions.
ThinCert® HTS migration assays in practice – we’ll let the data do the talking!
Using the new ThinCert® 96 well HTS insert, we’ve developed a high-throughput assay protocol to quantify migration of HT-1080 human fibrosarcoma cells. The results demonstrate that the assay is sensitive, accurate, and highly reproducible—from well-to-well, plate-to-plate and lot-to-lot. To see the methodology and data for yourself, download our latest application note below.
In the next and final article of this series, we’ll take a closer look at how our innovative HTS plate design helps you avoid three of the most common problems when implementing transwell assays for HTS.