Profiling Drug Effects in Patient-Derived Tumoroids Provide an In-Depth Understanding of Drug Sensitivity
How to streamline translational studies based on tumoroids
While a transition from two-dimensional (2D) tumor models to three-dimensional (3D) tumoroids is a highly translational approach to the study of cancer, handling and analyzing tumoroids can be a challenge. In this article, we look at how one group of researchers used a combination of a miniaturized platform and magnetic immobilization of tumoroids to examine the effects of anti-cancer drugs on breast cancer in the context of personalized medicine [1].
The study was based on tumoroids formed from primary cells isolated from a patient-derived breast cancer tumor, TU-BcX-4IC. This is a rare breast cancer subtype that represents a highly heterogeneous phenotype of breast cancer with high rates of metastasis, recurrence and chemoresistance. The value of these tumoroids as a model of predictive clinical outcome was assessed by analyzing their response to paclitaxel, romidepsin, trametinib, and cytarabine and comparing the results to what can be expected in patients.
Tumoroid analysis in a flowchip
Cromwell and coworkers used Pu·MA System® (Protein Fluidics, Inc.) to run automated sample processing [2]. In some experiments, tumoroids were incubated with NanoShuttle-PL and positioned on the bottom of the flow chip well by magnetic immobilization, thus centering them to facilitate imaging (Figure 1). Magnetic immobilization also simplified the handling of the small fragile tumoroids, which are sensitive to manipulations such as media exchange and supernatant sampling.
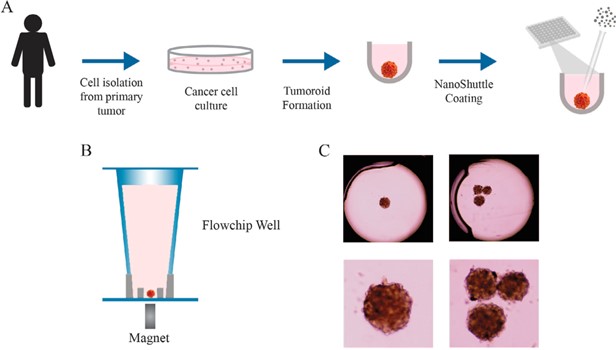
Figure 1:The process of tumoroid formation and loading into the flowchip. (A) Cancer cells were isolated from the primary tumor in a patient and cultured for 7–10 days.
Cells were then loaded into low-attachment plates where they formed tumoroids over 2–5 days. For some experiments, tumoroids were incubated with NanoShuttle-PL to magnetize them, assisting transfer to flowchips and subsequent immobilization (B). Immobilization simplified imaging of the tumoroids using both transmitted light (C) and in immunofluorescence experiments. From Figure 2, Cromwell et al, 2022 [1].
This approach enabled the researchers to monitor the effects of anti-cancer drugs on tumoroids held in the 3D flowchips using three types of analysis:
- Luminescence-based assays to determine cell viability
- Multiple time-point sampling of lactate secretion to measure metabolism
- Immunofluorescence with high-content imaging to determine the expression of E-cadherin and CD44 biomarkers as a measure of breast cell cancer number
Automated processing of a highly translational model
There were marked phenotypic changes in the tumoroids after drug treatment. Live cell staining decreased, dead cell staining increased, and tumoroids disintegrated to some extent. The tumoroids were most sensitive to romidepsin and trametinib, and significantly less sensitive to paclitaxel and cytarabine. This was consistent with the primary tumor response, indicating that the approach is highly translational.
The authors concluded that the ability of this approach to enable automated multi-parametric profiling of drug effects in patient-derived tumoroids and give insights into drug sensitivity of individual tumor types, makes it a promising approach for personalized medicine.
Request publication
Harnessing the potential of 3D cell culture
Watch an interview with Dr. Evan F Cromwell, CEO, President and Founder of Protein Fluidics Inc., talking to Dr Glauco Souza 3D cell culture expert at Greiner Bio-One, which highlights the benefits of combining magnetization to simplify the formation and manipulation of tumoroids with immobilization and analysis in microliter-scale flowchips.
Ready to enter the next level?
Please fill out this form and contact our experts today to find the perfect solution for you!
Don't miss our regular updates on scientific topics around 3D Cell Culture
References
[1] Cromwell EF, Sirenko O, Nikolov E, Hammer M, Brock CK, Matossian MD, Alzoubi MS, Collins-Burow BM, Burow ME. Multifunctional profiling of triple-negative breast cancer patient-derived tumoroids for disease modeling. SLAS Discov. 2022 Apr;27(3):191-200. doi: 10.1016/j.slasd.2022.01.006. Epub 2022 Feb 4. PMID: 35124274.[2] Cromwell EF, Leung M, Hammer M, et al. Disease modeling with 3D cell-based assays using a novel flowchip system and high-content imaging. SLAS Technol 2021;26:237–48.
