Magnetic 3D Bioprinting - New Possibilities in High-Throughput Screening
Magnetic 3D bioprinting (M3D) combined with cell-repellent surfaces is a superior technology for enabling the miniaturization and speed needed for HTS.
Content of this article:
- Culture miniaturization to enable and improve HTS in 3D
- Organoids without an exogenous extracellular matrix
- Enabling tissue complexity, co-culturing different cell types with M3D
Culture miniaturization to enable and improve HTS in 3D
There are still many unmet challenges when using 3D cell culture for high-throughput screening (HTS). A current hurdle is miniaturization needed to enable screening speeds to maximize efficiency and reduce costs. For cheaper, faster, and better assays in 3D, the miniaturization process requires outcomes better than 2D cell culture. As samples and volumes are scaled down, the sensitivity of instrumentation, such as the microplate reader and high-content imaging systems, must also evolve to provide reliable readouts using high-density plate formats.[1]
The published research by Fernandez-Vega et al.[2] presents the results of an HTS of 150K compound library where miniaturization was enabled by magnetic 3D bioprinting (M3D). This was the first report of high-throughput screening of this scale using clinically relevant primary pancreatic organoids from patient biopsies. Here, assay efficiency was achieved with a highly miniaturized and cost-effective methodology.
Miniaturization brings clear benefits in HTS, where automated assays with scaled-down volumes allow users to cut costs and speed screening times, enabling users to expedite the decision-making process. [1]
Organoids without an exogenous extracellular matrix
Organoid cultures are usually generated using exogenous extracellular matrix (ECM) components which can provide required physiologically relevant microenvironment and soluble factors. However, these products are often derived from a mouse tumor rich in extracellular matrix (ECM) proteins, carrying many drawbacks, including poor reproducibility and ethical considerations, as it requires the use of animals for production.
While there is the mischaracterization that organoids can only be generated using these animal-based extracellular matrix products, this is inaccurate. Organoids can also be produced without the use of animal-based ECM. Fernandez-Vega et al.[2] show that scaffold-free cell-cell interaction can enable the formation of organoid cultures that traditionally depend on exogenous extracellular matrices (ECMs). Here, magnetic 3D bioprinting is used to generate organoids for high-throughput screening (in 384 or 1536 well formats) of patient-derived pancreatic cancer with insightful outcomes.
Enabling tissue complexity, co-culturing different cell types with M3D
Interactions between cells of the same and different types and their extracellular environment, including the extracellular matrix, can direct cell viability, stem cell differentiation, and many other cell or tissue functions. Tissue or tumor microenvironment (TME) composition is essential to regulate cell morphology and physiology. The TME can dictate cancer cells to metastasize and to become more invasive. Furthermore, cancer tumors carry many cell types, including fibroblasts or cancer-associated fibroblasts (CAF), adipocytes, and immune and endothelial cells.
The cell-cell interactions and signaling between these cells are pivotal in cancer initiation, progression, and overall prognosis. For example, CAF can promote cancer progression and trigger inflammatory responses that can enhance or suppress cell proliferation. Read More
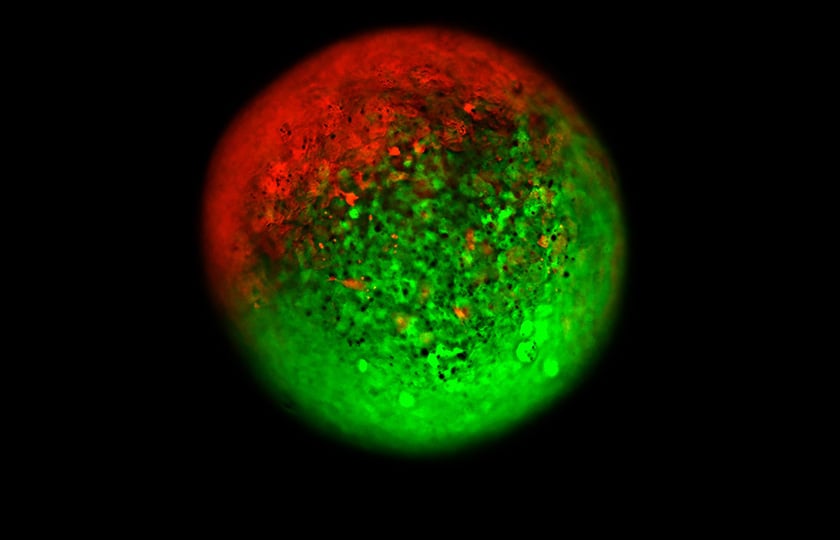
Although single cell type 3D cell culture provides tremendous value, combining multiple cell types is needed to extend predictivity and accuracy of compound screening. Fernandez-Vega et al.[2] demonstrated how M3D can facilitate the co-culture of primary cancer cells and CAFs from the same patient. The results show that cells brought together magnetically will organize spontaneously, where cancer cells (green) migrate to the outside and CAF localize in the center with organotypic morphology (Figure 1). These complex patient-derived organoids were then used to evaluate their sensitivity to chemotherapeutic agents and help to sort the heterogeneity effect on chemotherapy response for each of these samples.
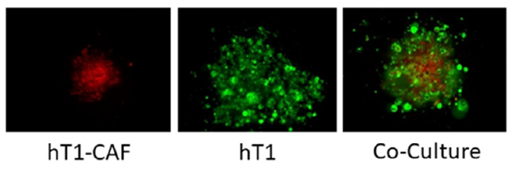
Image analysis to demonstrate how spheroids and co-culture spheroids look in the assay. Octadecyl Rhodamine B Chloride (R18) to stain the hT1-CAF cells and CellTracker Green to stain the hT1 cells and monitored their 3D structures at 96 hours post seeding at equal numbers of cells. Images were acquired at 10X using the ThermoFisher CellInsight.
Not only cancer research can benefit from increased complexity by co-culturing different cell types. In a separate study entitled "Scalable Approach Reveals Functional Responses of iPSC Cardiomyocyte 3D Spheroids," Burnham et al introduced a novel and scalable approach to assemble the co-cultures of cardiomyocytes (CMs) derived from induced pluripotent stem cells (iPSCs) with human primary cardiac fibroblasts using M3D. [3]
Here, M3D helped to overcome many of the limitations of traditional electrophysiology approaches using 3D cell cultures by improving reproducibility and accuracy by enabling ease of handling and higher throughput. By using magnetized cells, spheroids are accurately positioned magnetically onto electrodes, where both contractility (impedance) and extracellular field potentials (EFPs) were measured from the beating spheroids after automated compound dosing. [3] M3D can help to improve the predictiveness of cardiovascular efficacy and safety assays with relevant models and better electrophysiology readouts.
M3D is a superior technology for enabling the miniaturization and speed needed for HTS
References
[1] Maffia AM, Kariv I, Oldenburg KR. Miniaturization of a Mammalian Cell-Based Assay: Luciferase Reporter Gene Readout in a 3 Microliter 1536-Well Plate. Journal of Biomolecular Screening. 1999;4(3):137-142. doi:10.1177/108705719900400307
[2] Fernandez-Vega V, Hou S, Plenker D, Tiriac H, Baillargeon P, Shumate J, Scampavia L, Seldin J, Souza GR, Tuveson DA, Spicer TP. Lead identification using 3D models of pancreatic cancer. SLAS Discov. 2022 Apr;27(3):159-166. doi: 10.1016/j.slasd.2022.03.002. Epub 2022 Mar 17. PMID: 35306207.
[3] Burnham MP, Harvey R, Sargeant R, Fertig N, Haddrick M. A Scalable Approach Reveals Functional Responses of iPSC Cardiomyocyte 3D Spheroids. SLAS DISCOVERY: Advancing the Science of Drug Discovery. 2021;26(3):352-363. doi:10.1177/2472555220975332