Assessing a Model to Mimic Infection by Coronavirus
Model systems are invaluable in efforts to dissect the mechanisms involved in viral infection. Researchers have combined a coronavirus with low pathogenicity and a cell line grown as an organotypic culture with an air-liquid interface that could together help in generating data relevant to coronavirus infections, including COVID-19.
The need for surrogate cell models for respiratory diseases
The COVID-19 pandemic highlights the need for models that can be readily used to gain insights into the mechanisms of viral infection and replication. This was the focus of Gino Castillo and his colleagues at the Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, USA(1). Their work aimed at developing a model that could be used under low stringency laboratory conditions and yet generate data relevant to infections by more highly pathogenic coronaviruses such as SARS-CoV-2.
The virus they used, HCoV-NL63, is mainly associated with upper and lower respiratory tract infections in young children. Like SARS-CoV and SARS-CoV-2, HCoV-NL63 uses angiotensin-converting enzyme 2 (ACE2) as a receptor for binding to viral spike (S) protein and cell entry, which are essential steps in viral infection. While SARS-CoV-2 must be handled in Biosafety Level-3 (BSL-3) laboratories, HCoV-NL63 can be safely handled under BSL-2 conditions, which is clearly a major benefit in the study of disease mechanisms and therapeutics for SARS-like coronaviruses.
Towards an organotypic airway culture to recapitulate airway epithelia
For a model system to be of value in, for example, the screening of antivirals based on the detection and quantification of replicating virus, the virus must bind and enter target cells that support both viral gene expression and efficient virus release. Several cell lines are permissible to HCoV-NL63, including LLC-MK2, which is a standard immortalized cell line routinely used for virus isolation and propagation, together with vero cells, human renal epithelial cells, and primary human airway epithelial cells. However, since immortalized cells do not accurately mimic an infection in vivo, human respiratory epithelial cells (HRECs) are often used to improve the predictivity of experiments. They are the main target for infection and replication. While the cell line is important, the way it is cultured can also play a key role in mimicking infection. The researchers therefore compared three cell-based models in terms of cell susceptibility and replication efficiency with respect to HCoV-NL63:
1. LLC-MK2 cells grown as monolayers,
2. HRECs grown as monolayers, and
3. HRECs grown as organotypic air-liquid interface (ALI) cultures using ThinCert®.
The method they used for growing HRECs as ALI cultures using ThinCert® is described in more detail in the next article in this series. Subscribe to our blog series and stay up-to-date!
An air-liquid interface increases ACE2 expression and virus susceptibility
Some of the results from this study are shown in Figure 1 and give an insight into the overall relationship between susceptibility to the virus (HCoV-NL63 N-protein level) and the expression of the ACE2 receptor. Monolayers of LLC-MK2 cells had the greatest susceptibility and level of ACE2 expression, while HREC monolayers showed the lowest levels, and introducing an air-liquid interface to the HREC culture (ALI-HREC) increased these levels considerably.
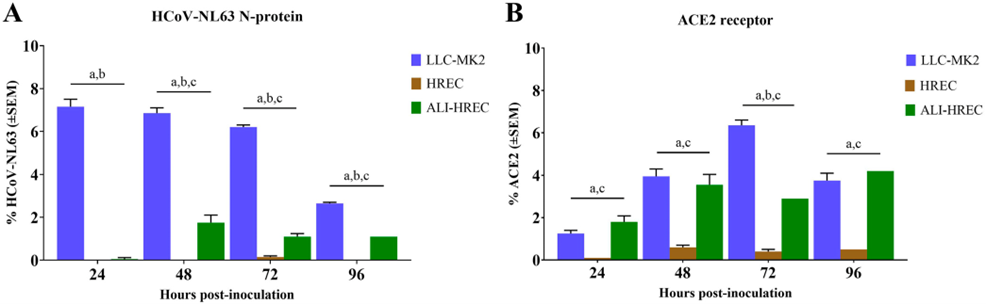
Figure 1: Flow cytometric analysis of LLC-MK2 cells, HRECs, and ALI-HRECs inoculated with HCoV-NL63 showing relative expression of (A) the HCoV-NL63 N protein, and (B) the ACE2 receptor protein. Cell percentages are expressed as mean ± standard error of the mean (SEM). Statistically significant differences (P < 0.01) are shown for (a), LLC-MK2 versus HREC; (b), LLC-MK2 versus ALI-HREC, and (c), HREC versus vALIHREC for each time point. From Figure 4, Castillo et al, 2022 (1), made available under the terms of the Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by/4.0/).
| Factor | LLC-MK2 | HEREC | ALI-HREC |
| Susceptibility | ++ | + | + |
| Replication efficiency | ++ | - | + |
| ACE2 expression | +++ | - | ++ |
| Main target of HCoV-NL63 | - | + | + |
While LLC-MK2 cells have high ACE2 expression levels that lead to high susceptibility to HCoVNL63, and support high replication efficiency, HRECs are the natural target for the virus. The transition from low complexity, HREC monolayers to organotypic HREC cultures with an ALI therefore increased ACE2 expression and HCoVNL63 replication efficiency. ALI-HREC cultures have also been successfully used to investigate other human coronaviruses, including HCoV-NL63, HCoV-229E, HCoV-HKU1, HCoV-OC43, SARS-CoV, Middle East respiratory syndrome-CoV (MERS-CoV), and SARS-CoV-2 (see 1).
The importance of ALI in mimicking conditions in respiratory epithelia
This study showed how introducing an ALI increases the relevance of a cell model that can be used under BSL-2 conditions by more closely mimicking the target tissue. In this study, the result was increased ACE2 receptor expression, which is a critical factor for susceptibility to the low pathogenic HCoV-NL63 viral infection and replication. Organotypic in vitro cell culture models aimed at mimicking respiratory epithelia should therefore include subpopulations of human primary respiratory epithelial cells that express ACE2 and recapitulate cell composition, structure, and functionality of the respiratory epithelia in vivo.
In the final article in this series, we will look in more detail at how ThinCert® was used to prepare the organotypic airway culture used in this study.
Ready to enter the next level?
Please fill out this form and contact our experts today to find the perfect solution for you!
Don't miss our regular updates on scientific topics around 3D Cell Culture
References
[1] Castillo G, Mora-Díaz JC, Nelli RK, Giménez-Lirola LG. Human Air-Liquid-Interface Organotypic Airway Cultures Express Significantly More ACE2 Receptor Protein and Are More Susceptible to HCoV-NL63 Infection than Monolayer Cultures of Primary Respiratory Epithelial Cells. Microbiol Spectr. 2022 Aug 31;10(4):e0163922. doi: 10.1128/spectrum.01639-22. Epub 2022 Jul 12. PMID: 35863002; PMCID: PMC9431431.