ThinCert® - A System for Studying SARS-CoV-2 Mechanisms
ThinCert® — a system for studying SARS-CoV-2 infection mechanisms and transport mechanisms: ThinCert®, a two-compartment system, can be readily used to prepare a cell model to mimic respiratory epithelia including an air-liquid interface for studies on virus infection. It can also be used to mimic a range of other in vivo situations including cell migration, interactions, lumen-lumen transport, and prepare models of vascularized tumors. The study of respiratory infections such as COVID-19 and intestinal diseases such as inflammatory bowel disease (IBD) is made possible by the availability of in vitro cell models to mimic the air-liquid or liquid-liquid interface of in vivo conditions. These models can be achieved by culturing relevant cell types in two-compartment membrane devices.
Exploring mechanisms at epithelial interfaces - air-liquid and liquid-liquid
Many biological processes occur at interfaces involving epithelia — for example at air-liquid interfaces in the respiratory tract, and at liquid-liquid interfaces involving epithelia of the intestinal tract. Cell-based studies of processes such as respiratory infection by viruses or transport mechanisms in the intestine require more than simple monolayer cell culture. Recapitulating the complexity and functionality of these interfaces in vitro relies on reproducing a two-compartment structure and function with relevant cell types in a way that simplifies laboratory manipulations such as buffer exchange and handling for analysis. We will look at examples that illustrate how ThinCert® has been used to generate advanced in vitro cell models to facilitate studies on coronavirus infection and intestinal transport.
Organotypic ALI airway culture models for respiratory infection research
The air-liquid interface plays a key role in the respiratory tract and simulating this has involved the culture of primary airway cells on microporous membrane scaffolds at the air-liquid interface (ALI) (1–6). Cell models including ALI have been prepared using a wide range of cells from nasal, bronchial, and alveolar epithelium (4, 5) and co-culturing can increase physiological relevance (6).
These differentiated, organotypic ALI airway models have several advantages compared to simpler models such as submerged or organoid cultures, including:
- They mimic in vivo structures and metabolic functions more closely.
- They can be dosed in a more relevant manner.
- Primary cells that undergo cellular differentiation can reproduce an in vivo–like transcriptional profile similar to that of the human airway epithelium that can play a key role in infection by certain human respiratory pathogens.
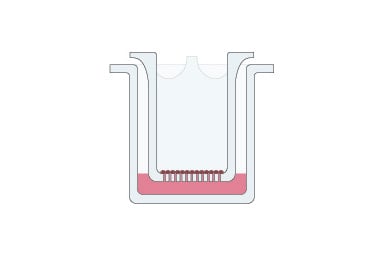
How to develop an organotypic airway culture to study coronavirus infection
ALI models can be used to screen for therapeutically relevant agents and their effectiveness on virus inactivation (6), and polarized ALI cultures are valuable as a tool for rapidly gaining information about infection, replication, and pathogenesis of the virus (7, 8).
Models based on coronavirus with lower pathogenicity could give insights into viral infection and replication under Biosafety Level-2 (BSL-2) conditions. This was the focus of Gino Castillo and his colleagues at the Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, USA (9).
The low-pathogenic virus they used, HCoV-NL63, is mainly associated with upper and lower respiratory tract infections in young children. Like SARS-CoV and SARS-CoV-2, HCoV-NL63 uses angiotensin-converting enzyme 2 (ACE2) as a receptor for binding to viral spike (S) protein and cell entry, which are essential steps in viral infection.
While the cell line is important, the way it is cultured can also play a key role in mimicking infection. The researchers therefore compared three cell-based models in terms of cell susceptibility and replication efficiency with respect to HCoV-NL63. These models involved LLC-MK2, a standard immortalized cell line used for virus isolation and propagation, and human respiratory epithelial cells (HRECs), the main target for infection and replication:
- LLC-MK2 cells grown as monolayers,
- HRECs as monolayers, and
- HRECs grown as organotypic air-liquid interface (ALI) cultures using ThinCert®
Monolayers of LLC-MK2 cells had the greatest susceptibility and level of ACE2 expression. Monolayers of HRECs, the natural target for the virus, had the lowest levels of ACE2 expression, but introducing an air-liquid interface to the HREC culture (ALI-HREC) increased these levels considerably. Introducing an air-liquid interface using ThinCert® therefore increased the relevance of the cell model together with HCoV-NL63 as a safe surrogate for the study of SARS-CoV-2 infection that can be used under BSL-2 conditions.
ThinCert® enables the generation of advanced model systems to study transport mechanisms
Transport studies are among the most frequent applications of ThinCert® cell culture inserts. ThinCert® enables the construction of a functional epithelium from individual cells to study the active transport of substances from one compartment through the epithelium to the other compartment. The cell culture insert with a porous membrane supports the generation of a polarized epithelium with tight junctions and basolateral and apical membrane compartments as the result of two phenomena:
- The insert membrane, which usually carries an extracellular matrix (ECM) treatment, can mimic the basement membrane
- The pores of the membrane allow the cells to take up nutrients from the basolateral side
Refining an intestinal epithelium model with co-culture
Recapitulating the in vivo situation of epithelia includes establishing a tight epithelium without trans-cellular leakage. The importance of leakage in the functioning of intestinal epithelia is particularly apparent in the case of the so-called “leaky gut” condition studied by Le and colleagues at the University Medical Center and Faculty of Medicine, University of Freiburg, Germany (10). They showed how ThinCert® can be used to generate advanced in vitro models for the study of transport through intestinal epithelia, including leaking that causes disease.
The “leaky gut” condition involves increased intestinal permeability caused by loose tight junctions of intestinal epithelial cell walls, allowing harmful substances such as pathogens and toxic digestive metabolites to pass from the gut into the bloodstream. This can lead to systemic inflammation and immune system activation, and chronic conditions such as autoimmune diseases, food sensitivities and allergies, asthma, and chronic inflammatory bowel disease (IBD).
Studies on IBD have frequently involved the Caco-2 cell line, a heterogeneous human epithelial colorectal adenocarcinoma cell line, as an in vitro cell model. The single-cell Caco-2 model can be enhanced by co-culture with the mucin-secreting HT29-MTX-E12 goblet cell line to more closely mimic conditions affecting permeability.
The IBD model can be refined to a new level by incorporating intestinal macrophages that, when activated, contribute to chronic intestinal inflammation. Le and colleagues therefore used ThinCert® to develop an advanced in vitro triple-culture model of the human intestine, consisting of Caco-2 cells, HT29-MTX-E12 mucus producing goblet cells, cultured on inserts in close contact with either differentiated human macrophage-like THP-1 cells, or primary human monocyte-derived macrophages obtained from PBMCs of healthy donors.
Conclusions
The investigation of mechanisms related to interfaces such as the air-liquid interface involved in respiratory disease and the liquid-liquid interface involved in intestinal transport is greatly simplified when in vitro cell models can be generated that closely mimic in vivo conditions. This can be achieved by developing models based on two-compartment systems such as ThinCert® and the co-culture of multiple cell types.
Find out more about how a transition from simple cell monocultures to differentiated, organotypic airway models with an air-liquid interface (ALI) and polarized epithelial cells give greater insight into the mechanisms involved in respiratory diseases.
Ready to enter the next level?
Please fill out this form and contact our experts today to find the perfect solution for you!
Don't miss our regular updates on scientific topics around 3D Cell Culture
References
[1] Cao X, Coyle JP, Xiong R, Wang Y, Heflich RH, Ren B, Gwinn WM, Hayden P, Rojanasakul L. Invited review: human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. In Vitro Cell Dev Biol Anim. 2021 Feb;57(2):104-132. doi: 10.1007/s11626-020-00517-7. Epub 2020 Nov 11. PMID: 33175307; PMCID: PMC7657088.[2] Adler KB, Schwarz JE, Whitcutt MJ, Wu R. A new chamber system for maintaining differentiated Guinea pig respiratory epithelial cells between air and liquid phases. Biotechniques 1987; 5:462–465
[3] Whitcutt MJ, Adlet KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988; 24:420–428
[4] Hasan S, Sebo P, Osicka R. A guide to polarized airway epithelial models for studies of host-pathogen interactions. FEBS J. 2018 Dec;285(23):4343-4358. doi: 10.1111/febs.14582. Epub 2018 Jul 2. PMID: 29896776.
[5] Silva S, Bicker J, Falcão A, Fortuna A. Air-liquid interface (ALI) impact on different respiratory cell cultures. Eur J Pharm Biopharm. 2023 Mar;184:62-82. doi: 10.1016/j.ejpb.2023.01.013. Epub 2023 Jan 22. PMID: 36696943.
[6] Baldassi D, Gabold B, Merkel O. Air-liquid interface cultures of the healthy and diseased human respiratory tract: promises, challenges and future directions. Adv Nanobiomed Res. 2021 May 6;1(6):2000111. doi: 10.1002/anbr.202000111. PMID: 34345878; PMCID: PMC7611446.
[7] Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727-733. doi: 10.1056/NEJMoa2001017. Epub 2020 Jan 24. PMID: 31978945; PMCID: PMC7092803.
[8] Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Feb 22;395(10224):565-574. doi: 10.1016/S0140-6736(20)30251-8. Epub 2020 Jan 30. PMID: 32007145; PMCID: PMC7159086.
[9] Castillo G, Mora-Díaz JC, Nelli RK, Giménez-Lirola LG. Human Air-Liquid-Interface Organotypic Airway Cultures Express Significantly More ACE2 Receptor Protein and Are More Susceptible to HCoV-NL63 Infection than Monolayer Cultures of Primary Respiratory Epithelial Cells. Microbiol Spectr. 2022 Aug 31;10(4):e0163922. doi: 10.1128/spectrum.01639-22. Epub 2022 Jul 12. PMID: 35863002; PMCID: PMC9431431.
[10] Le NPK, Altenburger MJ, Lamy E. Development of an Inflammation-Triggered In Vitro "Leaky Gut" Model Using Caco-2/HT29-MTX-E12 Combined with Macrophage-like THP-1 Cells or Primary Human-Derived Macrophages. Int J Mol Sci. 2023 Apr 18;24(8):7427. doi: 10.3390/ijms24087427. PMID: 37108590; PMCID: PMC10139037.
