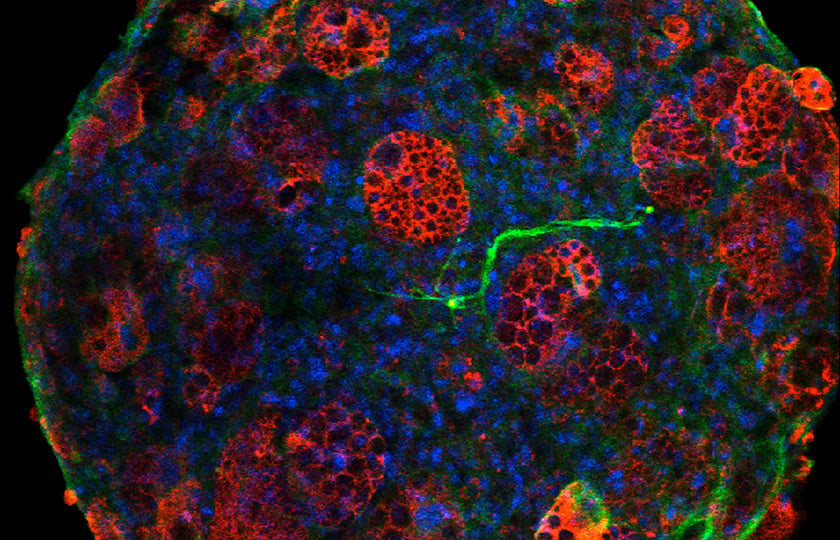
Development of High-Throughput Lacrimal Gland Organoid Platforms for Drug Discovery
New cell-based in vitro and in vivo technologies offer promising approaches for drug discovery in dry eye disease (DED) and potential human transplantation techniques for lacrimal gland (LG) tissue regeneration. In this article, we summarize the key findings of an exciting recent paper by Rodboon and colleagues [1] to give an overview of how the lacrimal gland organoid platform they developed provides robust lacrimal gland organoids for future potential high-throughput analysis and drug discovery. We also look at why magnetic three-dimensional (M3D) cell culture are enabling tools for stem cells and regenerative medicine.
What’s the cutting-edge research targeting LG organogenesis?
To research drugs against DED, mainly mouse models have been used so far. However, the transferability of mouse models to humans is quite limited because there are significant differences between the murine and human lacrimal glands. Porcine lacrimal gland bears more similarities to its human counterpart. Still, a comprehensive understanding of LG organogenesis, biology, and physiology during epithelial maturation is required for the development of the porcine LG organ in vitro. In a recent publication, Rodboon et al. discuss the steps of LG organogenesis in detail to apply to developing LG organoid platforms for various applications, including bioprinting and high-throughput screening [1].
What are the results?
Researchers have found that LG organoids provide a promising functional model for tissue rupture, a platform for drug screening, and potential clinical applications for DED.
The group already successfully generated innervated secretory epithelial organoids from human dental pulp stem cells with M3D Bioprinting and found that this platform is feasible for epithelial organoid bioprinting. Now, they have performed preliminary studies using M3D to generate LG organoids from primary cells isolated from mouse and porcine LG and found that this platform provides robust LG organoids for future potential high-throughput analysis and drug discovery.
M3D culture technology offers a consistent approach towards exocrine gland organoid biofabrication, particularly for the salivary glands and potentially for the LG as well. Hence, M3D can be a feasible in vitro platform for future high-throughput drug screening in exocrine gland organoids.
Why are magnetic 3D cell cultures enabling tools for stem cells and regenerative medicine?
The use of stem cells to produce organoids by 3D cell culture is a hot topic in current scientific debates. It is important to note that the production of organoids is highly dependent on growth factors, media types, and various supplements for the differentiation process, as organoids usually arise from stem cells.
However, 3D cell culture is not only used in stem cell research and discovery. Work with stem cells is equally relevant to regenerative medicine. In the differentiation process, researchers can use many tools for which M3D technology is valuable [2,3,4].
A good example of the success of regenerative medicine is the replication of salivary glands: For cancer patients, radiation is one of the most important types of treatment. Cancer can destroy the salivary gland, so that patients can no longer produce saliva after radiation and consequently suffer from a dry mouth, which greatly affects their quality of life.
Request the article
Adine et al. created mini salivary glands from organoids of stem cells, which they differentiated [5]. One mouse was exposed to irradiation, another was not treated and serving as a control. The scientists then implanted the organoids they had created with 3D cell culture into the animals and found that they worked in both animals.
This example shows that organoids clearly provide functional models for tissue rupture, drug screening, and diverse clinical applications.
Listen to our podcast with Dr. Glauco R. Souza, Director of Global Business Development & Innovation at Greiner Bio-One, where he reveals exciting insights regarding the biocompatibility of Nanoshuttle-PL, which is used to enable magnetic 3D cell culture by magnetizing the cells. For further details, you can also download our helpful whitepaper on this topic here.
Ready to enter the next level?
Please fill out this form and contact our experts today to find the perfect solution for you!
Don't miss our regular updates on scientific topics around 3D Cell Culture
References
[1] Rodboon T, Yodmuang S, Chaisuparat R, Ferreira JN. Development of high-throughput lacrimal gland organoid platforms for drug discovery in dry eye disease. SLAS Discov. 2022 Apr;27(3):151-158. doi: 10.1016/j.slasd.2021.11.002. Epub 2021 Dec 4. PMID: 35058190.
[2] Fang Y, Eglen RM. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017 Jun;22(5):456-472. doi: 10.1177/1087057117696795. Erratum in: SLAS Discov. 2021 Oct;26(9):NP1. PMID: 28520521; PMCID: PMC5448717.
[3] Hou S, Tiriac H, Sridharan BP, Scampavia L, Madoux F, Seldin J, Souza GR, Watson D, Tuveson D, Spicer TP. Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening. SLAS Discov. 2018 Jul;23(6):574-584. doi: 10.1177/2472555218766842. Epub 2018 Apr 19. PMID: 29673279; PMCID: PMC6013403.
[4] Tseng H, Gage JA, Raphael RM, Moore RH, Killian TC, Grande-Allen KJ, Souza GR. Assembly of a three-dimensional multitype bronchiole coculture model using magnetic levitation. Tissue Eng Part C Methods. 2013 Sep;19(9):665-75. doi: 10.1089/ten.TEC.2012.0157. Epub 2013 Feb 25. PMID: 23301612.
[5] Adine C, Ng KK, Rungarunlert S, Souza GR, Ferreira JN. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials. 2018 Oct;180:52-66. doi: 10.1016/j.biomaterials.2018.06.011. Epub 2018 Jun 12. PMID: 30025245.